New research highlights the complex task of Lyve-1, a key receptor that promotes the entry of immune cells into lymphatic vessels, and sheds light on its unique interaction with hyaluronan (HA). This study reveals an innovative sliding mechanism that allows immune cells to easily cross the endothelial junction. This can have a major impact on understanding health and the immune response of illness.
Lyve-1, or lymphatic endothelial receptor-1, has long been recognized for its role in assisting immune cells in translocating through lymphatic vessels. This movement is essential to maintain immune surveillance and inciting an appropriate immune response. Until recently, however, the exact mechanisms of how HA, an important glycosaminoglycan found on the surface of immune cells, interact with Lyve-1, are intersecting.
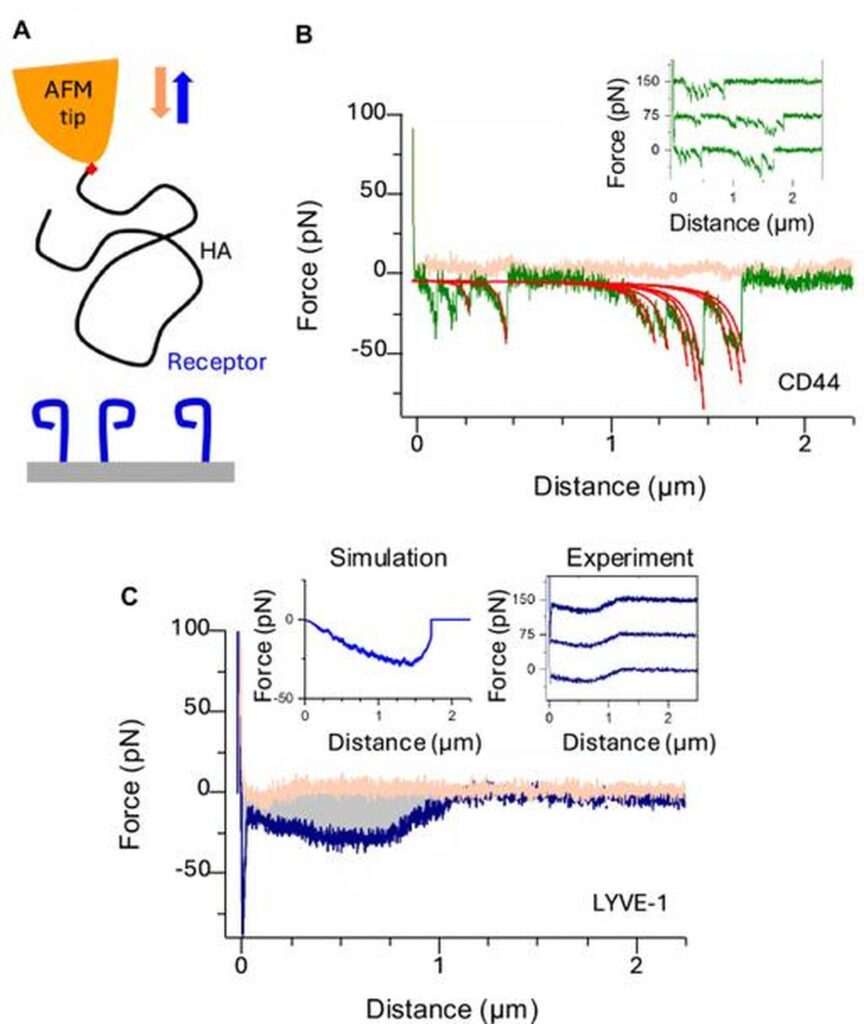
In the current study, we employed a combination of dynamic force spectroscopy (DFS) and X-ray crystallography to reveal the binding dynamics of the mouse and human Lyve-1 complex. This study illuminates the unique slide interaction Lyve-1 display with HA, allowing immune cells to reduce friction and transition from tissue to lymphoid capillaries. “These findings describe the mode of action of dedicated lymphatic invasion receptors and define clear, low tack adhesion interactions that allow migrating immune cells to slide through the endothelial junctions with minimal resistance,” the article author writes.
Until now, the process by which Lyve-1 binds to HA and promotes cell migration was not clearly understood. New insights highlight the importance of the non-reducing edge of the HA chain where Lyve-1 is preferentially involved. This preference enhances receptor function in lymphoid entry, as identified during experiments that revealed slide behavior.
In contrast to Lyve-1, closely related receptor CD44 operates through a mechanism that is characterized by individual binding rupture when binding to HA, compared to the cooperative nature of Lyve-1 interactions. The researchers said “The ability of the HA chain to move first and then form a dynamic H-bond with the surface aquatic layer is consistent with its function as a lubricating cushion around the sugar.” This demonstrates how Lyve-1 promotes efficient migration throughout the lymphatic endothelium.
The implications of these findings go beyond basic biological understandings. They could pave the way for innovative therapeutic strategies targeting immune cell trafficking. Enhanced or inhibited LYVE-1 function can affect how immune responses are regulated in a variety of diseases, from infection to cancer.
Importantly, this study also revealed the complex codependent nature of Lyve-1 and HA in promoting immune cell movement. Lyve-1 binds heavily on the structural integrity of the HA chain, suggesting that by manipulating HA availability, it changes the position of tissue immune cells, thereby modulating the immune response. As the authors conclude, ongoing research needs to elucidate how these different properties of Lyve-1 can be exploited for the development of interventions aimed at regulating immune function.
As we develop an understanding of immune mechanisms, Lyve-1 stands out as a promising candidate for targeted therapeutic development. To understand its unique binding properties and the mechanisms of its interaction, researchers need to further explore the role of receptors in both normal physiological and pathological conditions. In doing so, scientists can utilize these insights for strategic advances in immunotherapy.
This study not only bridges important gaps in understanding immune cell migration, but also highlights distinct functional mechanisms within the broader complexity of immune responses.